By year-end 2023, a significant breakthrough in slowing the progression of Alzheimer’s disease gained traction in the United States. Federal Drug Administration. Alzheimer’s disease is just one of many devastating neurological disorders that affect nearly 15% of the global population, highlighting the urgent need for further research into its causes and potential treatments. While advancements like the newly developed drug offer hope, there is still much to be learned about this complex condition and others like it.
“Mining the complexities of how the human brain adapts to mobile situations remains one of the most daunting tasks in neuroscience,” notes Lars Gjesteby, an expert at MIT’s Lincoln Laboratory. Excessive resolution in networked mind atlases has the potential to revolutionize our comprehension of complex issues by precisely identifying discrepancies between healthy and diseased brain patterns. Despite progress, hindrances persist due to a lack of suitable tools for visualising and navigating extremely large brain imaging datasets.
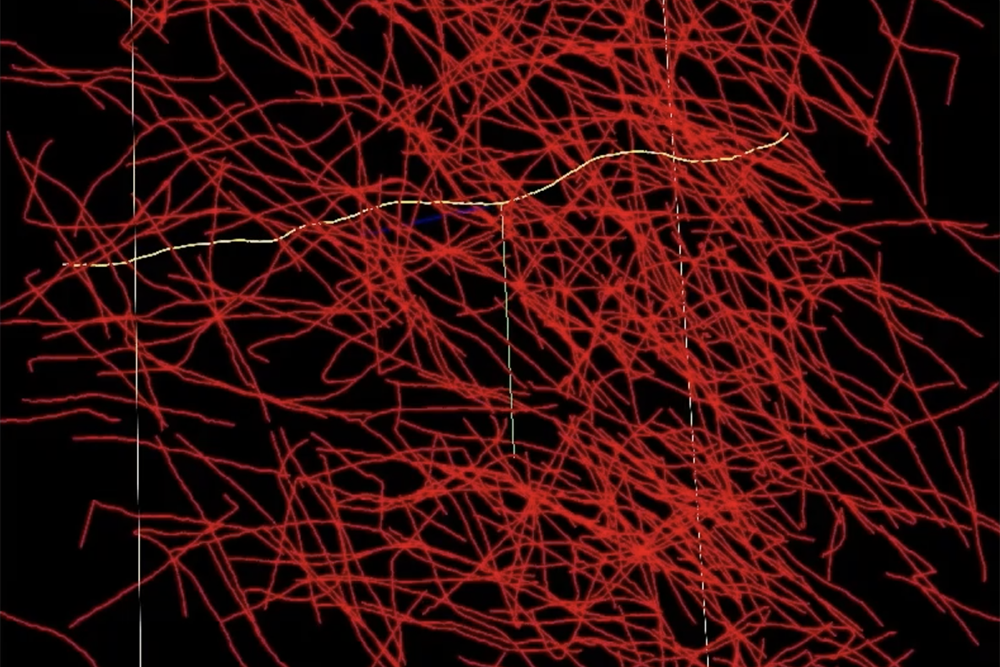
A networked mind atlas essentially represents a comprehensive mapping of the human brain, enabling the integration of structural information with neural function dynamics. To create detailed brain atlases, large amounts of brain imaging data require processing and precise annotation. Each axon, a slender fibre linking neurons, requires meticulous tracing, precise measurement, and comprehensive labelling with relevant details. Strategies for processing mind imaging information, akin to desktop-based software programs or manual-oriented tools, ought not be designed to handle human brain-scale datasets? Researchers often invest considerable time navigating through vast amounts of raw data.
The Gjesteby initiative aims to develop the Neuron Tracing and Energetic Studying Atmosphere (NeuroTrALE), a sophisticated software pipeline integrating machine learning, high-performance computing, and user-friendly access to advance brain mapping research. NeuroTrALE automates information processing, presenting results in an intuitive interface where researchers can edit and manipulate data to highlight, filter, and identify specific patterns.
Among NeuroTrALE’s distinctive features is its utilization of a machine-learning approach called lively studying. While NeuroTrALE’s algorithms excel at consistently labelling new data in accordance with existing brain imaging data, unfamiliar material may pose a risk of inaccuracies. Through active engagement in learning, users are empowered to correct mistakes by hand, thereby refining the AI’s performance for future similar instances. This combination of automation and guided labelling enables accurate information processing with a significantly reduced workload for the individual.
What would that even look like? According to Michael Snyder of the Homeland Choice Assist Methods Group at the laboratory, you’d witness a complex interplay of crisscrossed and overlapping genetic strains. When two yarn strands intersect, does this signify a 90-degree bend in one strand while the other continues straight upwards, or perhaps the opposite, where one strand follows a direct path while the other takes a perpendicular route? With NeuroTrALE’s engaging learning approach, users can manipulate individual threads of yarn once or twice, reinforcing their understanding as they master the algorithm’s ability to accurately project the strands’ movement. Without NeuroTrALE, individuals must tediously follow the complex neural pathways – akin to tracing individual fibers in a vast ball of yarn – every time they process information.”
As a direct outcome of NeuroTrALE, the significant labeling burden is alleviated from individuals, thereby enabling researchers to process an abundance of data more efficiently. The axon tracing algorithms leverage parallel computing to efficiently distribute calculations across multiple GPUs, thereby accelerating processing while ensuring scalability. With the aid of NeuroTrALE, a significant 90% reduction in computing time was achieved, enabling the processing of 32 gigabytes of data at an unprecedented pace, far surpassing conventional AI methods.
While the team verified that a significant increase in data volume does not necessarily correspond to a proportional boost in processing time. In this instance, the demonstration showed that a massive 10,000% increase in dataset size led to only a modest 9% and a notable 22% improvement in overall data processing time, using two distinct types of central processing models.
Benjamin Roop, an algorithm builder for the mission, notes that “manually labelling all of the axons in a single brain would require countless lifetimes” considering the 86 billion neurons forming 100 trillion connections within the human mind. This revolutionary instrument has the capacity to automate the construction of complex connectomes, not limited to a single individual, but applicable across multiple subjects. That opening the door to understanding mental health conditions at a fundamental level.
The NeuroTrALE mission was conceived as a collaborative endeavour jointly funded by Lincoln Laboratory and the Massachusetts Institute of Technology (MIT) campus-based research facility. To facilitate efficient data analysis, the Lincoln Lab team developed an innovative solution for the Chung Lab researchers to leverage and extract valuable insights from their vast amounts of brain imaging data streaming into the MIT-affiliated Lincoln Laboratory’s high-performance computing infrastructure. With its expertise in high-performance computing, advanced image processing, and sophisticated artificial intelligence, Lincoln Lab was uniquely positioned to tackle this complex challenge.
By 2020, the research team successfully deployed NeuroTrALE on the SuperCloud, allowing them to generate significant progress by 2022 when the Chung Lab began yielding tangible results from their collaborative efforts. Researchers employed NeuroTrALE to investigate the relationship between prefrontal cortex cell density and Alzheimer’s disease, finding that brains afflicted with the condition exhibited reduced cell density in specific regions compared to those without the illness. Researchers successfully placed a device that targets the areas of the brain where neurofibers tend to become entangled in Alzheimer’s-afflicted brain tissue, similar to those affected by the disease.
NeuroTrALE’s development has progressed steadily, thanks to ongoing support from Lincoln Laboratory and the National Institutes of Health (NIH), which have contributed to expanding its functional capacities. Currently, datasets are being integrated into a cutting-edge, open-source platform, powered by Google’s innovative software, to provide a seamless, web-based viewer for neuroscience data. NeuroTrALE offers real-time collaboration capabilities, enabling multiple users to simultaneously access, visualize, and edit shared information in a dynamic environment. Customers can generate and modify diverse geometric forms akin to polygons, fractions, and outlines to streamline annotation tasks, while also personalizing color displays for each annotation to distinguish neurons in densely populated regions?
“NeuroTrALE provides a platform-agnostic, end-to-end solution deployable via containers across standalone, digital, cloud, and high-performance computing environments,” notes Adam Michaleas, an expert in high-performance computing from the laboratory. What enables seamless integration within the neuroscience community is the tool’s capacity to facilitate real-time collaboration through data visualization and concurrent content analysis, thereby significantly enhancing the expertise of the tip individual.
To align with the objective of sharing analytical insights, the team’s goal is to transform NeuroTrALE into a fully open-source tool that anyone can utilize seamlessly. According to Gjesteby, the type of instrument required to realize the ultimate goal of comprehensively mapping the human mind for purposes of analysis and ultimately enhancing drug development is crucial. As a grassroots movement, the collective aims to democratize access to critical information and sophisticated algorithms, ensuring they’re freely available to everyone.

