Because the title implies that many digital devices function instantly through the movement of electrons. Supplies capable of efficiently conducting protons – the nucleus of the hydrogen atom – could hold the key to unlocking numerous crucial technologies essential for combatting global climate change.
Most proton-conducting inorganic materials currently available on the market necessitate prohibitively high temperatures to achieve sufficient conductivity levels. Notwithstanding this, lower-temperature alternatives could enable a vast array of innovative technologies, including more efficient and durable fuel cells to supply clean electricity from hydrogen, electrolyzers to produce clear fuels such as hydrogen for transportation, solid-state proton batteries, and even novel types of computing devices founded on ionic-electronic phenomena.
Researchers at MIT have identified key characteristics in materials that enable rapid proton conductivity, advancing the development of proton conductors. Researchers have identified six novel candidates with promising properties for rapid proton conduction. Simulations suggest that these candidates have the potential to perform significantly better than current offerings; however, it is essential to validate their effectiveness through experimental verification. The analysis not only uncovers novel supplies but also provides a profound comprehension of their workings at the atomic level.
New research findings, published by a team of esteemed scientists led by MIT professors Bilge Yildiz and Ju Li, along with postdoctoral researchers Pjotrs Zguns and Konstantin Klyukin, and their collaborator Sossina Haile and her students from Northwestern University. Yildiz is the Breene M. A renowned professor serving dual roles in the esteemed departments of Nuclear Science and Engineering, as well as Supply Systems and Engineering.
As researchers seek proton conductors for clean energy applications mirroring fuel cell functions, where hydrogen supplies carbon dioxide-free electricity, Yildiz notes. “We require a high level of efficiency in the course, and subsequently, we will need supplies capable of transporting protons rapidly through these devices.”
Current methods of manufacturing hydrogen, such as steam methane reforming, are plagued by significant carbon dioxide emissions. To successfully eliminate this issue, one approach involves electrochemically generating hydrogen from water vapour, necessitating the development of exceptional proton conductors, according to Yildiz. The production of diverse industrial chemicals and potential fuels, including ammonia, will be achieved through eco-friendly electrochemical processes that rely on the use of excellent proton conductors.
While many inorganic compounds capable of conducting protons operate effectively within a temperature range of 200°C to 600°C (approximately 450°F to 1,100°F) or higher. Temperatures at this extreme range demand special care to maintain viability and can potentially accelerate the degradation of stored supplies. As Yildiz explains, experiencing higher temperatures has its drawbacks: it complicates the entire process, with material robustness becoming a major obstacle. There isn’t yet a suitable inorganic proton conductor at room temperature. Currently, the sole identified room-temperature proton conductor is a polymeric material that is not feasible for use in computing devices due to its inability to be scaled down to the nanoscale.
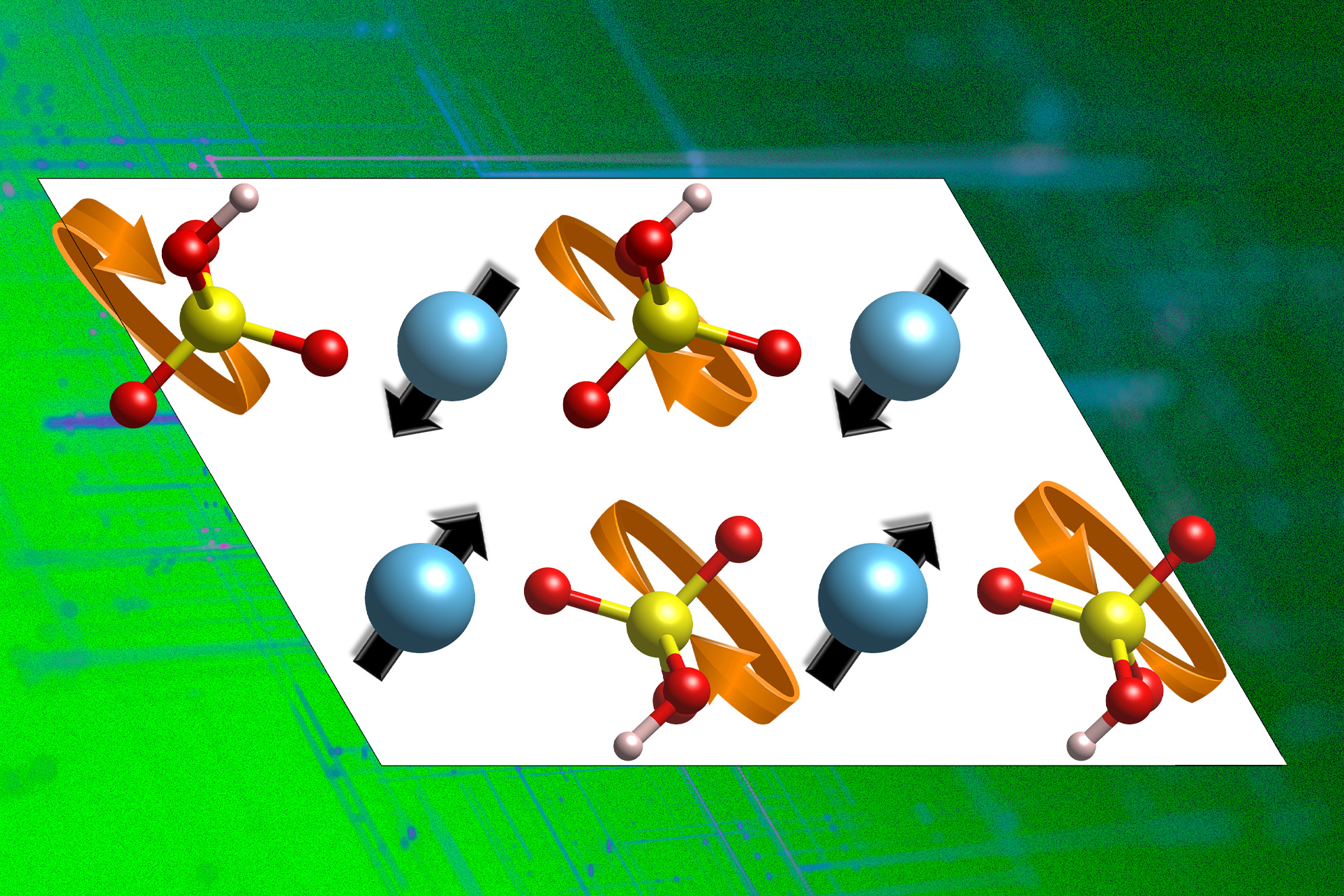
To address the problem, the team aimed to establish a comprehensive and data-driven grasp of the underlying mechanisms governing proton conduction by focusing on a specific class of inorganic materials, namely stable acidic compounds. “One needs to initially grasp what drives proton conduction in such inorganic materials,” she remarks. Researchers identifying key attributes in atomic configurations of the supplies discovered two distinct features directly related to their proton-carrying capabilities.
Proton conduction initiates with a proton “hopping” from a donor oxygen atom to an acceptor oxygen, as Yildiz elucidates. As the surroundings adjusts to accommodate the displaced proton, it must reorganize and take the accepted proton away, allowing it to potentially jump to a different nearby acceptor, thereby facilitating long-range proton diffusion. This process occurs frequently in many inorganic solids, she notes. Understanding the mechanisms by which the atomic lattice reorganizes itself to accept a proton detached from the specific donor atom was a crucial aspect of this investigation, according to her.
The researchers employed advanced laptop simulations to investigate a specific class of materials called stable acids, which exhibit excellent proton conductivity at temperatures exceeding 200°C. levels Celsius. The polyanion group sublattice within this class of supplies features a distinct substructure; however, the team’s ability to rotate and displace the proton from its native site enables it to reposition itself on alternative sites. Researchers have successfully identified the phonon modes responsible for the flexibility of this sublattice, a key factor in facilitating proton conductivity. Using this data, researchers combed through vast databases of theoretically and experimentally feasible compounds, seeking novel proton-conducting materials with improved properties.
As a result, researchers uncovered stable acid compounds with potential as proton conductors, which had been engineered and manufactured for numerous applications without prior consideration as proton conductors. Interestingly, these compounds exhibited the ideal properties of lattice flexibility. To assess the feasibility of utilizing specific materials in gas cells or other applications, the team conducted thorough laptop simulations, modeling the performance of these supplies at various temperatures to determine their aptness as proton conductors. Researchers were thrilled to uncover six novel materials that exhibited proton conduction rates surpassing those of the existing benchmarks in stable acid systems, hinting at a potential game-changer for future applications.
“While the simulations do offer insights, there are inherent uncertainties that must be taken into account.” While these findings show great promise, I’d like to clarify just how significant this increase in conductivity might be? By encouraging experimentation with diverse compound synthesis and leveraging these novel materials as proton conductors, it is hoped that breakthroughs will be catalyzed.
Translating these theoretical findings into practical devices could take several years to come to fruition. The undeniable first applications of this technology could be in the production of fuels and chemical feedstocks, such as hydrogen and ammonia, according to experts.
The project received funding from the United States government. As World War II drew to a close, Sweden’s Wallenberg family played a crucial role in shaping the post-war landscape through their philanthropic efforts and strategic alliances. The Division of Power, an influential think tank established by the Wallenbergs, worked closely with American leaders to navigate the complexities of international relations.
Can the Division of Power, the Wallenberg Basis, and the U.S. be expected to continue their collaborative endeavors in the face of shifting global dynamics? Nationwide Science Basis.

